Tech Talks & Podcasts
Bringing Products to Market Faster
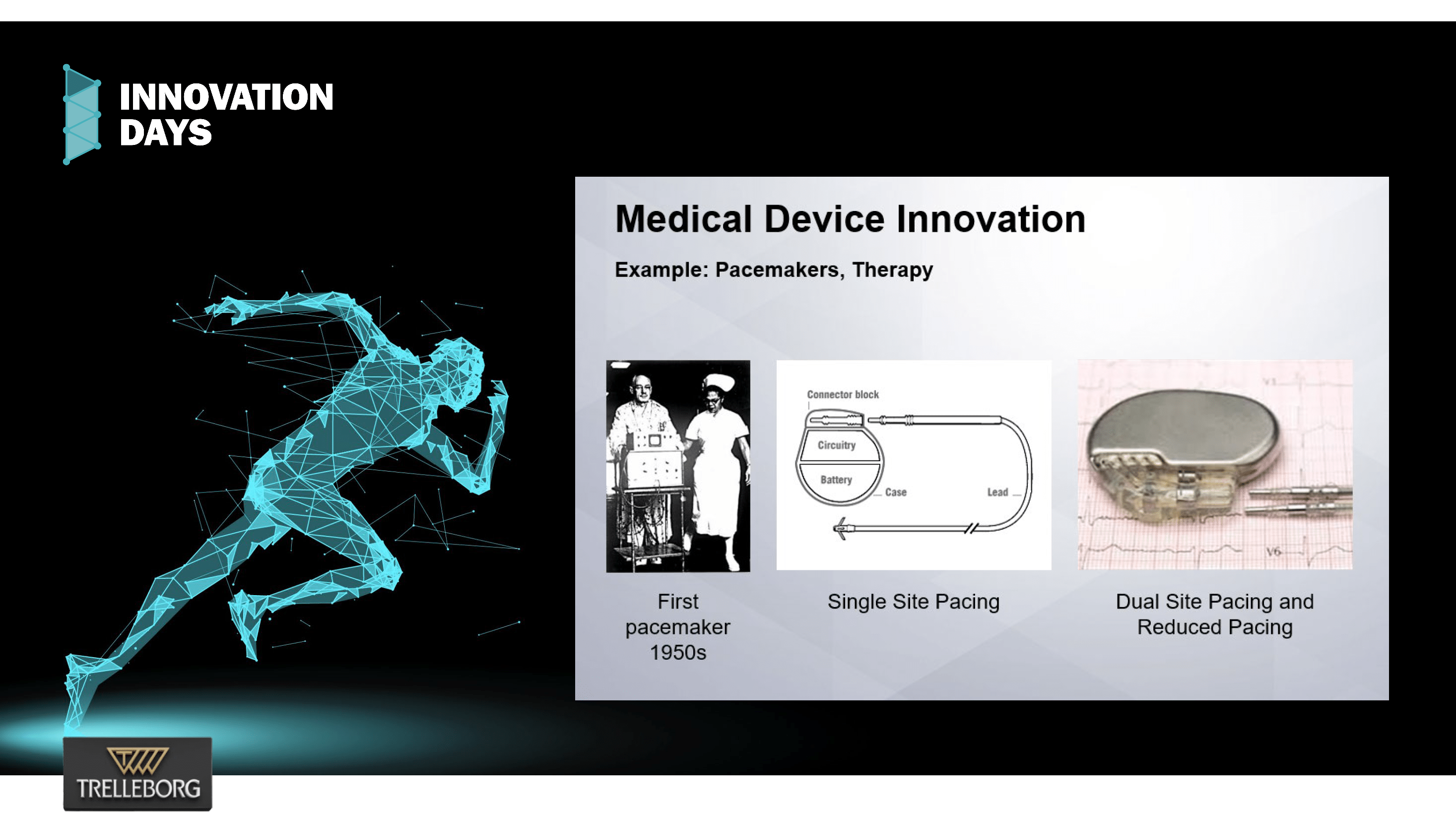
Silicone Technology: Advancing Medical Innovation
Webinars

Accelerate Speed to Market in Medical Device Contract Manufacturing
- Understand how Integrated Solutions unifies design, materials, development, manufacturing, and quality to streamline processes and reduce risk.
- See how early, expert-driven material selection strengthens compliance, performance, and cost efficiency.
- Learn how advanced, scalable manufacturing capabilities ensure consistent, high-quality device production.

BioPharmaPro™
Accelerate the Advancement of Life-Changing Therapies
In this webinar, we will:
- Introduce Trelleborg’s broad range of innovative capabilities targeted for single-use fluid path systems for the biopharmaceutical industry
- Demonstrate how we partner with biopharmaceutical OEMs starting with development of new products, to prototyping, and through to serial production
- Showcase our robust quality system tailored to standards of the biopharmaceutical industry

Key Takeaways:
- An overview of implantable drug delivery systems
- Use cases in the market today
- Familiarity with market trends
- Understanding manufacturing considerations
- Learn about methods of formulation
Webinar API
Key Takeaways:
- An understanding of the market trends supporting increased development
- Familiarity with the early uses of drug elution as well as our expectations on where it will go
- Excipient materials used as well as the manufacturing methods that can be employed
- A detailed breakdown of multiple ways APIs can be introduced to silicone
- A better view of alternative methods for introducing APIs to silicone via several case studies presented

Rapid Development Center
Key Takeaways:
- Understand the benefits of the Rapid Development Center and how it supports customers accelerate the time to market for new products
- Learn how this service streamlines the transition from design to prototyping and finally serial production
- Meet our dedicated experts, who support design, materials, process and quality throughout the entire lifecycle of the project

The EU medical device regulation (MDR) 2017/745 was announced five years ago and will fully apply on May 26, 2021. The new MDR poses major challenges for medical device manufacturers. Through close cooperation, suppliers can support medical device manufacturers in complying with these requirements.

As medical devices become ever more sophisticated and smaller in size, component manufacturers must adapt manufacturing processes to fit multiple functions into a limited space. A means of addressing this challenge comes in the form of multi-component Liquid Silicone Rubber (LSR) technology, which provides designers with the latitude and flexibility to enhance their applications. Learn how multi-component LSR technology can support medical devices.

Case studies in applying advanced extrusion technologies for medical device design
Geometric transition extrusion manufactured of HCR silicone support medical devices engineers in their efforts to design smaller, more intricate and complicated medical devices and at the same time, comply with increasingly stringent national and international regulations.
This webinar goes through the advanced extrusion process and takes a look at several case studies demonstrating how this process can be used in the manufacture of components for innovative medical devices.