Trelleborg Exhibits Innovations in Medical Device Components at Pharmapack
Showcasing the Rapid Development Center
At the show, Trelleborg will highlight its latest global capability to help healthcare and medical customers expedite their new product development process. This offering, called the Rapid Development Center, will help minimize production costs, quicken development time, and ensure superior-quality products.
>> Find out more about the Rapid Development Center
Linda Muroski, President of Trelleborg Global Healthcare & Medical and Marketing Americas, says: “The Rapid Development Center will accelerate our customers’ time to market and help them achieve their mission of improving patients’ lives. This service comprises several core competencies critical to medical device and pharmaceutical component development, including design, consultation, toolmaking, prototyping, high-precision machining, silicone molding, thermoplastic molding, automation, assembly, and secondary operations.”
State-of-the-art manufacturing
By using Trelleborg’s manufacturing facilities, customers have access to raw material traceability, Class 7 cleanrooms, and established validation processes. These facilities are ISO 13485:2016 and ISO 9001 certified and meet requirements from the Food and Drug Administration and European Medical Device Regulation.
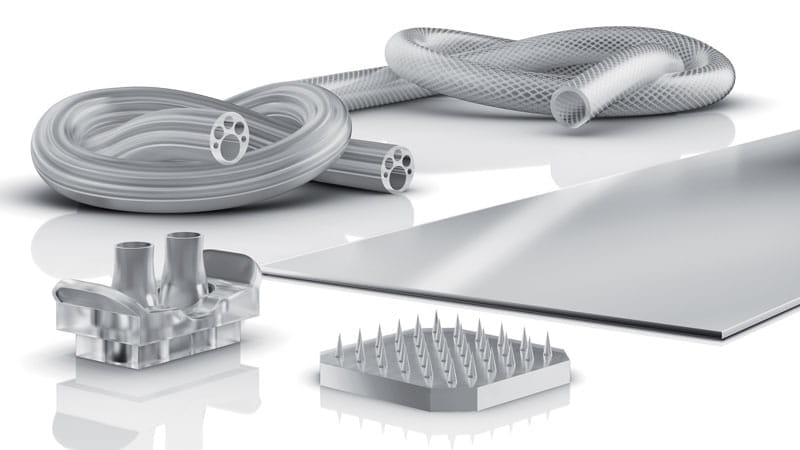
Specialty tubing & GeoTransTM extrusion
Visitors to the booth will also see an extensive selection of Trelleborg’s specialty extruded tubing and hose products. Tubes are available in a full range of sizes and are custom designed with customers to meet their individual application needs.
Andrew Gaillard, Global Director of Trelleborg Healthcare & Medical, says: “We engineer novel extrusion solutions for our customers’ specific tube and hosing applications. Examples include multi-lumen, silicone foam extrusions, and kink-resistant tubing for a variety of critical applications. Additionally, Trelleborg’s GeoTransTM technology enables the production of extrusion profiles that can change throughout the length of the extrusion, supporting unique, customer-specific designs.”
Advanced molding & micromolding capabilities
Trelleborg will also showcase its molded parts that are used in sealing solutions for medical device and drug-delivery applications. Utilizing in-house precision tooling and expertise, Trelleborg creates custom silicone and thermoplastic molded parts with tight tolerances, while the need for rapid product development is met with quick turnaround prototypes. Additionally, attendees will be able to see Trelleborg’s advancements in multicomponent and micromolding capabilities.
Gaillard says: “One of our most well accepted capabilities is the combination of Liquid Silicone Rubber (LSR) with plastics. Commonly referred to as 2C or 2-shot, multicomponent injection molding allows our LSR experts to employ highly sophisticated tool and process engineering to develop innovative solutions that combine two or more individual materials into one fully bonded, robust component.”
Drug-eluting silicone sheeting
Finally, attendees at Pharmapack will see Trelleborg’s silicone sheeting and drug delivery capabilities. These highlight the continued evolution of technologies enabling the development of novel solutions for patient care.
“In recent years, we’ve seen a spike in demand from our customer-partners for devices that incorporate an active pharmaceutical ingredient for targeted delivery of a therapy. Whether challenged with a wound-care application that requires wafer-thin silicone gel films with a drug element incorporated, or an innovative approach to cancer treatment with unique manufacturing challenges, our engineering teams are consistently eager to partner with customers to help solve interesting and complex problems,” concludes Gaillard.