Developing and Testing Materials for Medical Applications
At Trelleborg Medical Solutions, we bring your vision for medical devices and biopharmaceutical equipment to life by focusing on materials support. Our polymer and silicone experts work closely with customers to develop the optimum material compound to meet application requirements.
Customers are involved in material development at every stage, from initial understanding of operating conditions and concept development through to prototyping and testing. This holistic approach streamlines every phase, greatly enhancing device development and patient care, ensuring comprehensive support whether you're starting with an idea or scaling to mass production.
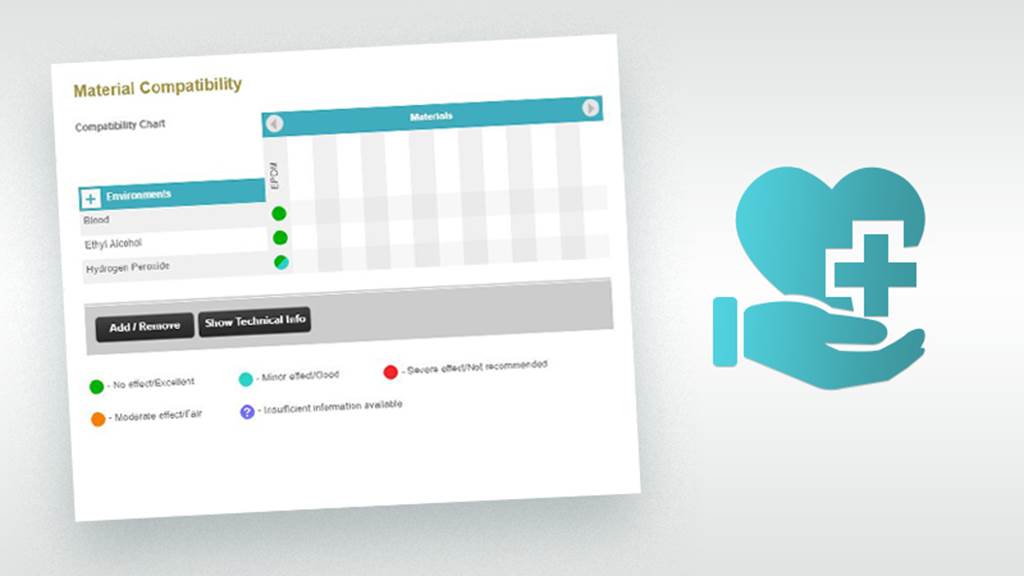
Chemical & Sterilization Compatibility

No single material is suitable for all medical application environments. Highly inert materials such as polytetrafluoroethylene (PTFE), perfluoroelastomer (FFKM) and silicone rubber inherently stand up to aggressive chemical and sterilization conditions with little or no damage. Other materials are specifically formulated and tested for compatibility with specific environments; establishing the degree of attack, how much of each property is lost and where the application boundaries lie for each material.
Importance of Chemical & Sterilization Compatibility:
- Materials available for a wide range of healthcare and medical applications
- Offer optimum performance in the most demanding environments
- Extend product life
- Lower overall cost of production
- Materials and products withstand multiple cleaning and sterilization cycles
- Any potential contamination from product deterioration is avoided
- Minimizes downtime
- Maximizes mean-time between planned maintenance
Increasing Demands for Cleaning and Sterilization
Sterilization is a key factor for many medical applications and the need for faster, more effective sterilization is leading to ever more aggressive sterilization methods. Clean-In-Place (CIP) and Sterilize-In-Place (SIP) processes provide increased efficiency because devices and systems do not need to be dismantled for cleaning.
Through extensive testing, Trelleborg Medical Solutions has identified the optimum materials for use in the most commonly used CIP and SIP media. Our medical solutions engineers partner with customers to select the most appropriate material and product for their application to balance cost with performance.
Material Compound Development

The development of polymer materials blends the science of understanding material chemistry and application conditions with the art of balancing a wide range of performance characteristics, to produce the optimum material for each unique customer need.
Trelleborg Medical Solutions material development experts are located in our manufacturing units and R&D centers globally. Constantly keeping ahead of new developments in thermoplastic and elastomer technology, they ensure that we can offer the most appropriate material for any application, however demanding.
Materials are selected and developed to ensure:
- Compatibility with a wide range of media
- Optimization for a balance of performance, processability and component life
- Maximized material performance to provide longer life, lower friction, operation at higher temperatures
- Suitability for robust, high quality manufacturing
- Accreditation to required standards and regulations
- An evolving portfolio to meet the changing needs of the healthcare and medical industry
Of particular importance to the healthcare and medical industry are silicone and PTFE based materials. With decades of experience in both of these key materials, Trelleborg Medical Solutions offers a wide portfolio of compounds to suit every application condition and, when required, all relevant standards and approvals.
Materials Testing for Medical Applications

Our Trelleborg Medical Solutions R&D centers are equipped with world-class material testing and analytical laboratories to ensure that our materials meet the requirements of your application’s environment. We test materials to:
- Confirm the material compatibility with application environments
- Ensure robust and long-lasting materials
- Continuously expand our portfolio of material compounds to meet the changing needs of the Medical industry
Our R&D centers are equipped with world class material testing and analytical laboratories to ensure that our materials are capable of performing in a wide range of environments. Capabilities include:
- Mechanical Testing
- Thermal Analysis
- Fluid Compatibility
- Microscopy
- Tribological Testing
Test facilities support recommendations of materials for customer applications, development of new materials, characterization of materials for Finite Element Analysis (FEA), failure analysis and the achievement of relevant industry material certifications.