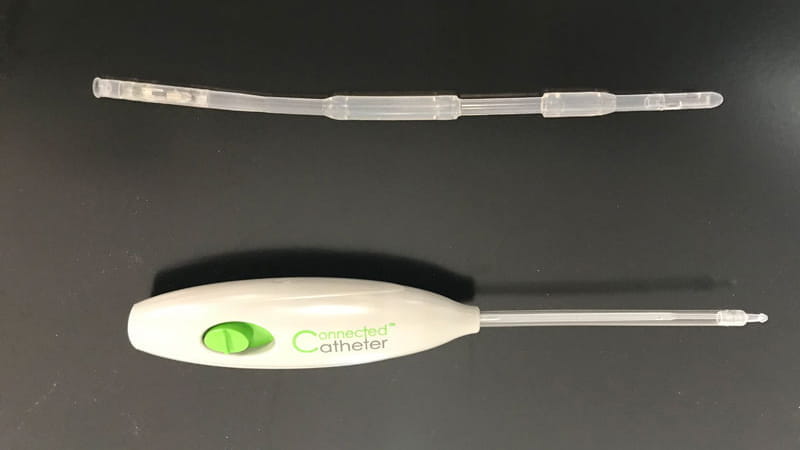
Connected Catheter System – Spinal Singularity, Inc.
Application: Fully internal, extended use, smart catheter system offering daily incontinence control to people with paralysis
Product type: Catheter and magnetically driven impeller
Spinal Singularity, Inc. was founded in 2015 by a paralyzed military veteran to design and develop a solution for bladder control among paralyzed people of all backgrounds. Spinal Singularity, Inc. needed supply chain management support in order to get the product to market.

Spinal Singularity, Inc. came to Trelleborg Healthcare & Medical because in addition to our ability to provide molded silicone parts and tubing, they recognized the value of:
- Our quality systems for medical products
- Our capabilities in assembly process development
- Our Design for Manufacturability (DFM) experience and processes
- The fact that we manufacture most of the catheter components in-house
- Our ability to act as a full-service partner where we can manage any part or all of the supply chain
- Our ability to support the company’s delivery schedule
Spinal Singularity, Inc. thinks of Trelleborg Healthcare & Medical as an extension of their manufacturing and engineering teams.

The final product is a urological catheter, a catheter insertion/removal tool and a control module.
At Trelleborg Sealing Solutions Tustin, we manufacture the catheter insertion/removal tool and the catheter. The catheter enables fluid flow from the bladder by a magnetically driven impeller at the end of the catheter, allowing the user to control when they empty their bladder.
The Connected Catheter System is currently in clinical trials and is expected to be commercially available in late 2019.
CAUTION: Investigational device. Limited by Federal (or United States) law to investigational use.